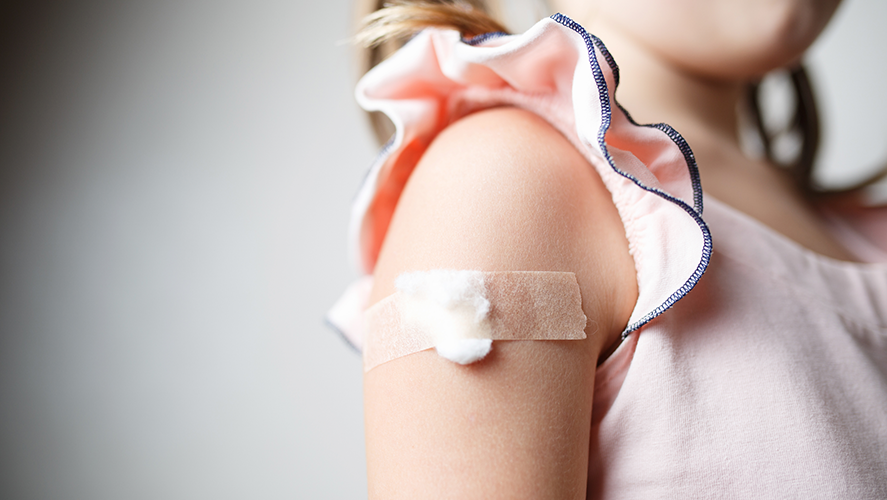
An FDA advisory panel has officially endorsed the Pfizer COVID-19 vaccine for children ages 5-11.
The panel made a unanimous decision on Tuesday, which now goes to the FDA for its final ruling, due sometime in the coming days.
If approved, the vaccine would be a smaller dose than the one given to adults – just 10 micrograms compared to 30 in the full vaccine. Just like the adult vaccine, it would be given in two doses, three weeks apart.
NBC News Special Report:
FDA advisory panel recommends Pfizer's Covid vaccine for emergency use in kids ages 5 to 11. https://t.co/EJGDy70cNOhttps://t.co/nBJo5fUTGW
— NBC News (@NBCNews) October 26, 2021
Has your family made a decision on what you’ll do once vaccines are available for younger children? Is there any chance the FDA rejects the panel’s advice?










